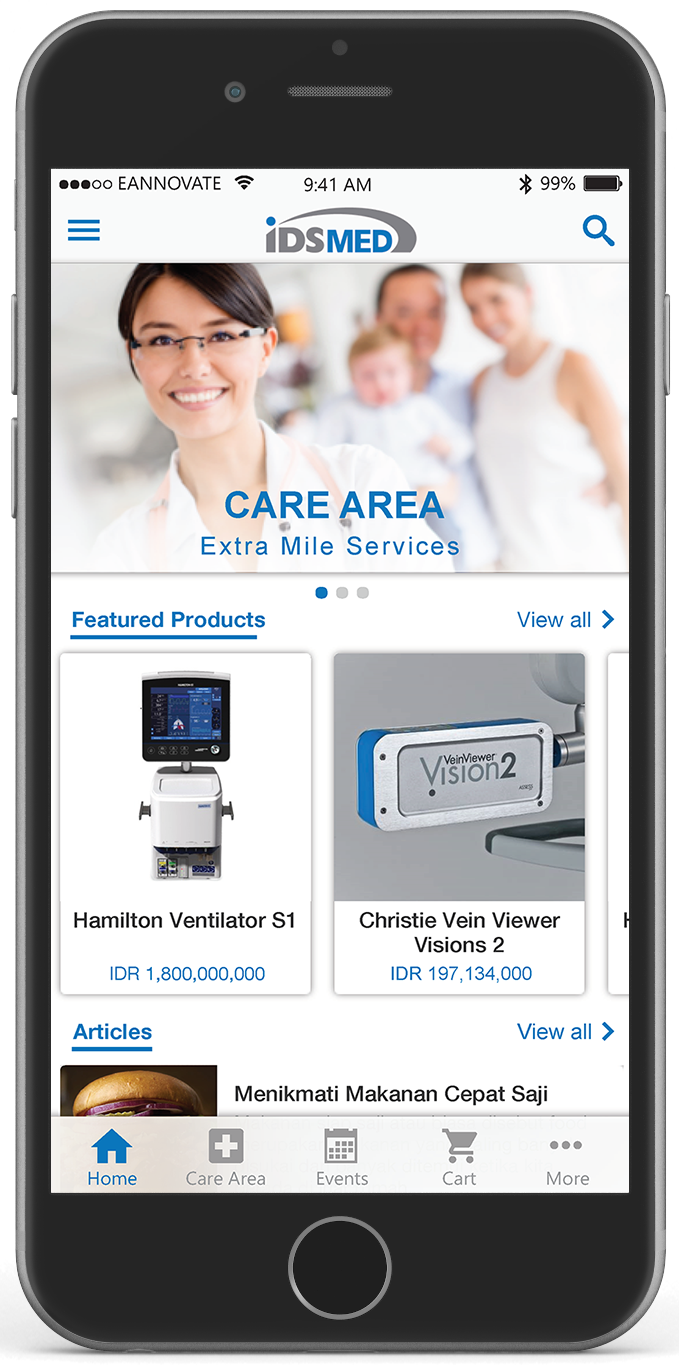
- ABOUT US
- Medical Specialty Care
- OUR SERVICES
- PARTNERS
- IDSMED LEARNING ACADEMY
- InnoQ
- News
- Talent & Jobs
- LOG IN / SIGN UP
+62 21 2567 8989
- English change
- Home
- About Us
-
Medical Specialty Care
- Aesthetic
- Anesthesiology & Analgesia
- Bio-Medical Services
- Cardio Vascular
- Dental
- Diagnostic Imaging
- Ear, Nose & Throat
- Emergency Care
- Gastroenterology
- General Surgery
- Geriatric Medicine
- Healthcare Education
- Infection Control
- Intensive Care
- Laboratory
- Medical Consumables
- Medical IT
- Neurology
- O&G and Peri-Natal
- Oncology
- Opthalmology
- Orthopedic
- Patient Support System
- Physio & Rehab
- Primary Care
- Respiratory Care
- Surgical Workplace
- Wound Management
- Our Services
- Partners
- idsMED Learning Academy
- News
- Talent & Jobs
- Login / Sign Up